Abstract
Background: D14 BM Bx are routinely performed to determine the need for immediate reinduction in patients with AML; however, the prognostic value of D14 BM Bx remains controversial and has not been established in the setting of ELN risk classification. We performed a retrospective review of AML patients who underwent intensive induction chemotherapy and determined the prognostic impact of D14 BM Bx stratified by ELN risk class on overall survival (OS) and relapse-free survival (RFS).
Methods: We included AML patients who underwent an intensive induction regimen (7+3) from January 2009 to December 2016 and had a D14 BM Bx. Characteristics for patients with residual disease (RD), defined as either ≥ 20% cellularity and/or ≥ 5% blasts, and no evidence of disease (NED) on D14 BM Bx were compared using Mann-Whitney test or chi-square analyses. Univariable and multivariable Cox proportional hazard regression models were used to estimate the hazard ratios (HRs) for mortality and relapse. For all survival analyses, patients were censored at time of transplant.
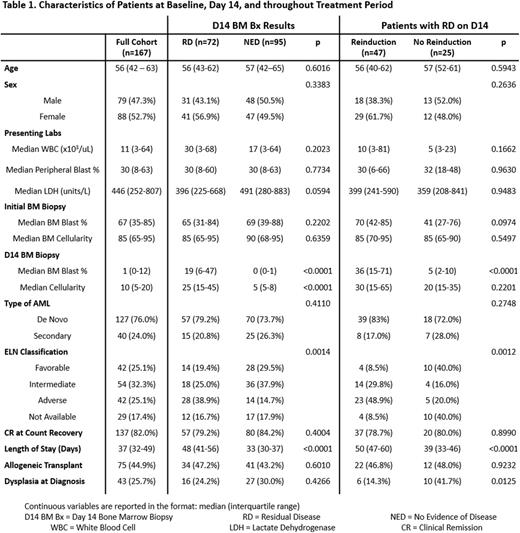
Results: The baseline characteristics of the study population (167 patients) are displayed in Table 1. Ninety-five patients (57%) attained NED status on D14 BM Bx, and 72 patients (43%) had RD. The only statistically significant difference in baseline characteristics was that NED patients had more favorable ELN classification (p < 0.0001).
There was no statistically significant difference in the complete remission (CR) rates between those with NED vs RD on D14 BM Bx (84.2% vs 79.2%; p = 0.4004). Of those with evidence of RD, 47 patients (65%) received reinduction therapy and 25 patients (35%) did not. Those who received reinduction therapy were more likely to have a less favorable ELN class (p = 0.0405) and a higher median blast percentage at day 14 than those who did not (36% vs 5%; p < 0.0001). There was no difference in the rates of CR at count recovery between those who received reinduction and those who did not (p = 0.8990).
There was no significant difference in median OS between patients with NED and RD on D14 BM Bx (3.8 years vs. 1.4 years; p = 0.0975); however, among patients who achieved CR, those with NED at D14 did have an increased median RFS (vs RD, 2.5 years vs. 0.6 years; p = 0.0038). Notably, there was no statistically significant difference in median OS or RFS in patients with NED vs RD at D14 when survival analysis was stratified by ELN risk group. When comparing those with NED and those with RD who did not receive reinduction therapy, there was no significant difference in OS (3.8 years vs NE; p = 0.9421) or RFS (2.5 years vs 1.1 years; p = 0.4473).
In unadjusted Cox regression model, OS was not significantly affected by D14 BM status (HR = 1.6; 95% CI 0.9 - 2.7; p = 0.1003), but risk of relapse was significantly increased among patients with RD (vs NED; HR = 2.1; 95% CI 1.3 - 3.6; p = 0.0046). Among those with RD at D14, reinduction was associated with an increased risk of all-cause mortality (vs no reinduction; HR = 2.4; 95% CI 1.4 - 4.2; p = 0.0026) and relapse (vs no reinduction; HR = 2.9; 95% CI 1.7 - 5.1; p = 0.0002). On multivariable analysis that incorporated age, sex, and all significant variables from the univariable models, mortality risk was expectedly significantly higher among patients with intermediate and adverse ELN risk groups. Importantly, there was no evidence of higher mortality risk seen in patients with RD on D14 BM Bx vs NED (HR = 1.1; 95% CI 0.4 - 3.0; p = 0.8625) or those that received reinduction therapy (vs no reinduction; HR = 1.5; 95% CI 0.6 - 4.2; p = 0.4072). Similarly, there was no evidence of higher relapse risk seen in patients with RD on D14 BM Bx vs NED or those that received reinduction therapy vs those that did not.
Conclusion: In this large retrospective cohort, similar clinical outcomes for CR, OS, and RFS were seen irrespective of D14 BM status even when stratified by ELN risk group cohort. There were also no appreciable differences in CR, OS, or RFS between those with RD that received reinduction therapy and those that did not. Limited by its retrospective nature, this study nevertheless challenges the prognostic value and clinical utility of D14 BM status, which merits more robust evaluation in a prospective randomized trial format.
Disclosures
Kremyanskaya:BMS: Research Funding; Chimerix: Research Funding; Incyte: Consultancy, Research Funding; Ionis: Research Funding; Kura: Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Research Funding; Protagonist Therapeutics: Consultancy, Research Funding; Kronos: Research Funding. Silverman:Takeda: Research Funding; Celgene/BMS: Research Funding; Gilead/Forty-Seven: Research Funding. Bar-Natan:Incyte: Research Funding; BMS: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding. Mascarenhas:Kartos: Consultancy, Research Funding; Janseen: Research Funding; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Forbius: Research Funding; Prelude Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy; Sierra Oncology: Consultancy; GSK: Consultancy; Merck: Research Funding; PharmaEssentia: Consultancy, Research Funding; Galecto: Consultancy; Geron: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Research Funding; Merus: Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Imago: Consultancy; Roche: Consultancy, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.